We are efficiently advancing three clinical-stage programs with differentiated mechanisms of action for cancer and immune diseases, providing multiple opportunities to deliver new medicines to patients
Soquelitinib (CPI-818)
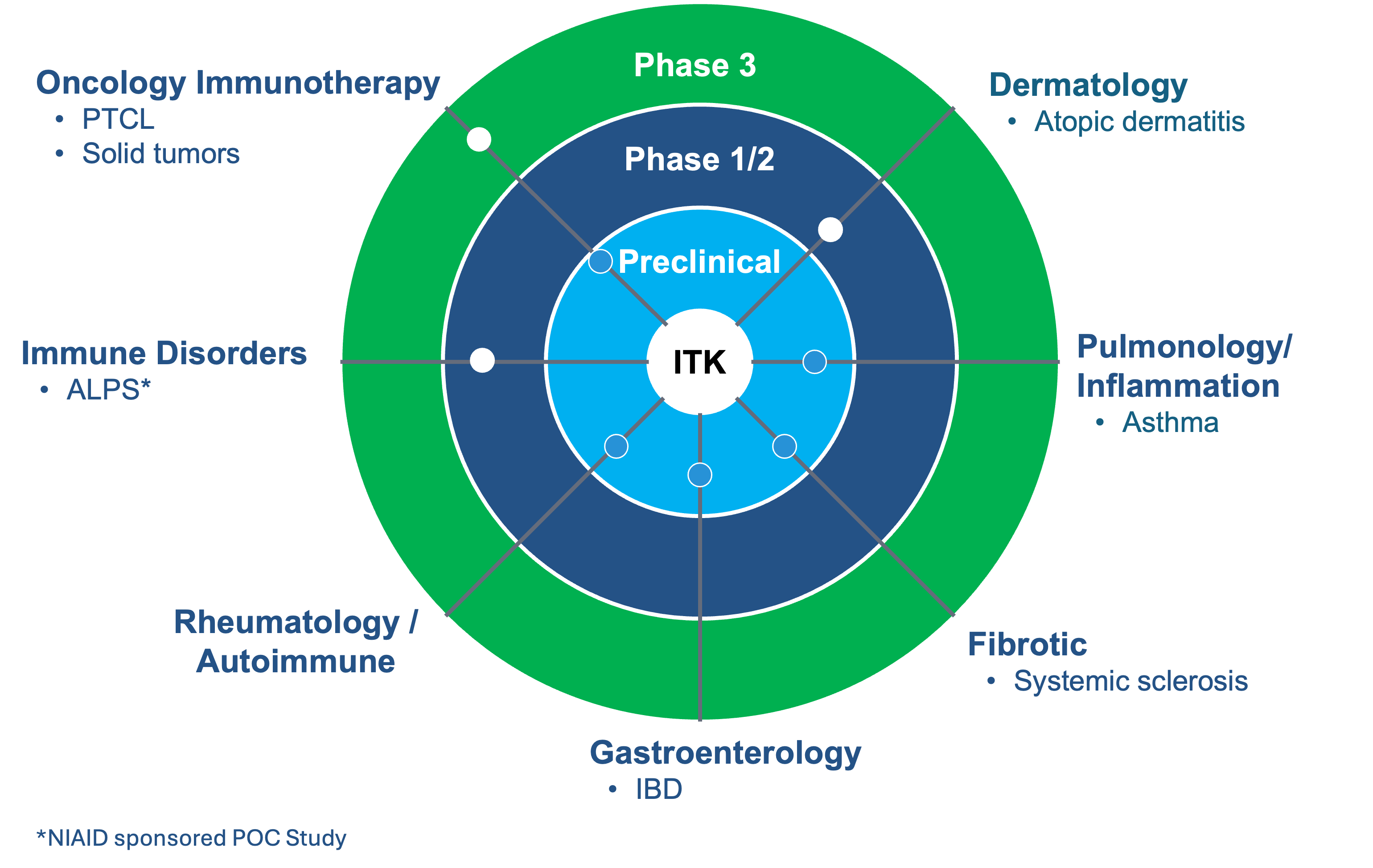
Soquelitinib is an oral, small molecular drug that selectively inhibits ITK (interleukin-2-inducible T cell kinase) and has the potential to provide a platform opportunity with broad applicability across lymphomas, solid tumors, and immune diseases. ITK is an enzyme expressed predominantly in T cells and that plays a role in T cell and natural killer (NK) cell lymphomas and leukemias, as well as in normal immune function. Soquelitinib binds to ITK and modulates T cells, similar to the way Imbruvica (ibrutinib) binds to BTK and modulates B cells for the treatment of B cell lymphomas and autoimmune diseases. In addition, preclinical data suggests that ITK inhibition has the potential to treat a variety of solid and hematological cancers based on a novel immunotherapy mechanism of action.
The optimal dose of soquelitinib has the potential to induce the activation, differentiation and expansion of T cells to TH1 helper cells while blocking the deployment of TH2 cells (TH1 skewing). Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. In addition, soquelitinib’s novel mechanism of action is believed to increase the infiltration of CD8 T effector cells into tumors, increase cytolytic capacity of CD8 cells and reduce and reverse T cell exhaustion. We believe the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with T cell lymphomas, leukemias and solid tumors, along with immune diseases, including atopic dermatitis and asthma.
Corvus and its partner in China, Angel Pharmaceuticals are conducting a Phase 1/1b trial in patients with refractory T-cell lymphomas that was designed to select the optimal dose of soquelitinib and evaluate its safety, pharmacokinetics, target occupancy, immunologic effects, biomarkers and efficacy. Interim data from the Phase 1/1b clinical trial of soquelitinib for T cell lymphoma demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, and identified a dose that maximally affects T helper cell differentiation. Corvus is conducting a Phase 3 clinical trial with soquelitinib in patients with relapsed/refractory peripheral T cell lymphoma (PTCL), a Phase 1 clinical trial in patients with moderate to severe atopic dermatitis and a Phase 2 clinical trial in patients with autoimmune lymphoproliferative syndrome (ALPS), a rare genetic disease. The Company is planning to conduct a Phase 1b/2 clinical trial in patients with metastatic renal cell cancer (RCC).
ITK: Focused on Target and Broad Indication Potential
Ciforadenant (CPI-444)
Corvus is a leader in the development of precisely targeted therapies targeting the adenosine pathway. Ciforadenant is a small molecule antagonist of the adenosine A2A receptor, a key step in the adenosine pathway leading to immunosuppression in the tumor microenvironment. By precisely targeting and blocking A2A receptors on immune cells, ciforadenant may unleash their cancer-killing properties.
Results from a Phase 1/1b clinical trial demonstrated that ciforadenant was active alone and in combination with atezolizumab in patients with advanced, refractory renal cell cancer (Fong et al., Cancer Discovery 2020). The study also describes the Adenosine Gene Signature, a novel biomarker, which was found to be beneficial in identifying patients most likely to respond to therapy.
An open-label Phase 1b/2 trial of ciforadenant in first line therapy for metastatic renal cell cancer in combination with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) is being conducted in collaboration with the Kidney Cancer Research Consortium (KCRC). The trial design is based on Corvus’ preclinical research published in 2018 in Cancer Immunology Research that demonstrated impressive antitumor control and cures in several animal models using ciforadenant in combination with anti-CTLA-4 and anti-PD1.
Mupadolimab (CPI-006)
Mupadolimab is a unique anti-CD73 antibody that blocks the production of adenosine in the tumor microenvironment. It also binds to B cells resulting in their activation and differentiation into antibody producing plasma cells. These attributes have the potential to augment both cellular and humoral immunity to cancers. Corvus has completed a phase 1 study in cancer patients and mupadolimab is now being evaluated in non-small lung cancer by its partner in China, Angel Pharmaceuticals.
For more information on our pipeline, please see our Annual Report available in the SEC Filings section of the website.
Our Science
We are developing ITK inhibition as a new approach to control the immune system in order to treat immune diseases and cancer
Join Our Team
Contribute your talent, expertise and desire to learn here at Corvus, where you can help improve the lives of people with immune diseases and cancer